Recycling cathodes; new phase change material; graphene girders.

Recycling cathodes
Nanoengineers at the University of California San Diego developed an energy-efficient recycling process that restores used cathodes from spent lithium ion batteries. The process involves harvesting the degraded cathode particles from a used battery and then boiling and heat treating them. In new batteries built with the cathodes, charge storage capacity, charging time and battery lifetime were all restored to their original levels.
Less than five percent of used lithium ion batteries are recycled today.
“Think about the millions of tons of lithium ion battery waste in the future, especially with the rise of electric vehicles, and the depletion of precious resources like lithium and cobalt–mining more of these resources will contaminate our water and soil. If we can sustainably harvest and reuse materials from old batteries, we can potentially prevent such significant environmental damage and waste,” said Zheng Chen, a professor of nanoengineering at UC San Diego.
The recycling method works for lithium cobalt oxide cathodes. The method also works on NMC, a popular lithium cathode containing nickel, manganese and cobalt, which is used in most electric vehicles.
The method involves first collecting cathode particles from spent lithium ion batteries. Researchers then pressurize the cathode particles in a hot, alkaline, solution containing lithium salt–this solution can be recycled and reused to process more batches. Afterwards, the particles go through a short annealing process in which they are heated to 800 C and then cooled very slowly. New cathodes were constructed with the regenerated particles.

Used cathode particles from spent lithium ion batteries are recycled and regenerated to work as good as new. (Source: David Baillot/UC San Diego Jacobs School of Engineering)
As a lithium ion battery wears out, the cathode material loses some of its lithium atoms. The cathode’s atomic structure also changes such that it’s less capable of moving ions in and out. The recycling process restores both the cathode’s lithium concentration and atomic structure back to their original states.
Overall, the recycling process uses 5.9 megajoules of energy, equivalent to the energy in about three quarters of a cup of gasoline, to restore one kilogram of cathode material.
To optimize the process for industrial scales, the team is planning to work with battery companies in Asia. A particular area of improvement is the cathode harvesting step, which is currently a manual process. Researchers are working on simplifying this step so that the entire process is industrially viable. The team is also working on a process to recycle used anodes.
New phase change material
Researchers at Tohoku University, the National Institute of Advanced Industrial Science and Technology (AIST), and Hanyang University, proposed a new phase change material, Cr2Ge2Te6, with electrical characteristics that behave differently to those of conventional materials. The team says the new material allows a drastic reduction in power consumption for data-recording in non-volatile random access memory.
Phase change random access memory (PCRAM) has been proposed as a next generation practical non-volatile memory. The principle of PCRAM operation relies on the change in electrical resistance between high resistance amorphous and low resistance crystalline states in phase change material.
Ge-Sb-Te (GST) is well known as a phase change material for PCRAM applications. GST can operate at high speed but has poor data retention at high temperatures (~ 85C) and needs a large amount of power for data recording.
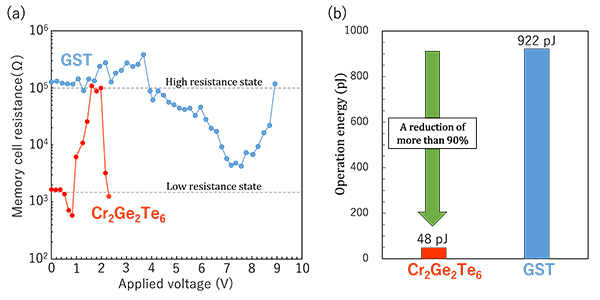
(a) Memory cell resistance vs. applied voltage curves in Cr2Ge2Te6 and GST memory cell. (b) Comparison of operation energy between Cr2Ge2Te6 and GST. (Source: Shogo Hatayama/Tohoku University)
The new Cr2Ge2Te6 phase change material exhibits an inverse resistance change from low resistance amorphous to high resistance crystalline states. The researchers demonstrated that the Cr2Ge2Te6 can achieve a reduction of more than 90% in power consumption for data recording compared to using a conventional GST memory cell.
Simultaneously, Cr2Ge2Te6 was found to combine a faster operation speed (~30 ns) and a higher data retention property (over 170C) than conventional materials. According to the team, comparison with other reported materials indicates that Cr2Ge2Te6 can break the trade-off relationship between data retention and operation speed.
Graphene girders
Researchers at the University of Warwick found a way to replace graphite in the anodes of lithium-ion batteries using silicon, by reinforcing the anode’s structure with graphene girders. This could more than double the life of rechargeable lithium-ion based batteries and also increase the capacity delivered by those batteries, the team says.
While silicon has ten times the gravimetric energy density of graphite, performance issues limit its commercial exploitation. Due to its volume expansion upon lithiation silicon particles can electrochemically agglomerate in ways that impede further charge-discharge efficiency over time. Silicon is also not intrinsically elastic enough to cope with the strain of lithiation when it is repeatedly charged, leading to cracking, pulverisation and rapid physical degradation of the anode’s composite microstructure. This contributes significantly to capacity fade, along with degradation events that occur on the cathode.

This is a diagram showing how the FLG flakes can prove very effective at preserving the degree of separation between the silicon particles with each battery charge cycle. (Source: University of Warwick)
The team’s approach uses a new anode mixture of silicon and a form of chemically modified graphene. The researchers created anodes that were a mixture of 60% micro silicon particles, 16% few-layer graphene (FLG), 14% Sodium/Polyacrylic acid, and 10% carbon additives, and then examined the performance (and the changes in structure of the material) over a 100 charge-discharge cycles.
“The flakes of FLG were mixed throughout the anode and acted like a set of strong, but relatively elastic, girders. These flakes of FLG increased the resilience and elasticity of the material greatly reducing the damage caused by the physical expansion of the silicon during lithiation. The graphene enhances the long range electrical conductivity of the anode and maintains a low resistance in a structurally stable composite,” said Melanie Loveridge, a Senior Research Fellow in WMG at the University of Warwick.
The FLG flakes also proved effective at preserving the degree of separation between the silicon particles, reducing the change silicon particles become electrochemically welded to each other when charging, a phenomena which degrades the battery’s life and power output.
The team is continuing work on the anode, including optimizing the electrode research, scaling up and manufacturing pouch cell li-ion batteries.
Leave a Reply